Baking Soda and Vinegar
Reaction and Demonstrations
"The baking soda and vinegar reaction
has been used in so many FUN ways!"
Baking soda and vinegar are two common materials found in almost every household. That, plus the fact that all the starting and finishing materials are non hazardous and safe, is why this is one of the first chemical reactions that many people are exposed to.
In fact, this vinegar reaction, has been widely used from public school science classes right up to the university level courses to analyze and demonstrate chemical reactions.
Some of the ways this versatile reaction has been used at home or at school involve:
- Describing a chemical reaction.
- Propelling a rocket with baking soda and vinegar.
- Making a bubble bomb.
- Demonstrating a volcano.
- Demonstrating the stoichiometry of a chemical reaction.
- Analyzing the reaction's change in temperature, pH and mass.
Baking soda, a pure chemical called sodium bicarbonate, has the chemical formula:
When dissolved in water baking soda separates into sodium (Na+) and bicarbonate ions (HCO3- ):
Vinegar, a weak (5%) solution of acetic acid in water, partially dissociates into hydrogen ( H+) and acetate ions (CH3COO-):
The reaction between baking soda and vinegar is actually two reactions, an acid base reaction followed by a decomposition reaction.
When the two ingredients are mixed, hydrogen ions ( H+) from the vinegar react with the bicarbonate ions (HCO3- ) from the baking soda to form a new chemical called carbonic acid (H2CO3).
The carbonic acid thus formed then immediately decomposes into carbon dioxide gas (CO2)and water (H2O).
It's this carbon dioxide gas that you see bubbling and foaming as soon as you mix baking soda and vinegar together.
Using the molecular structures of only the components involved, the chemical reaction can be written:

The overall reaction however, is often written as follows:
You can build your own rocket, fueled by baking soda and vinegar, out of common household items or you can purchase a science kit designed to launch a plastic rocket 100 feet into the air.


- Remove the lid from the empty film canister then pack it tightly with baking soda.
- Add into the film canister about two teaspoons of vinegar.
- Carefully place the lid onto the canister and snap it closed.
- Turn the film canister upside-down, place it on the ground and stand back.
The canister will shoot up into the air within a few seconds. This is due to the pressure buid-up as the carbon dioxide gas is produced. If it doesn't launch, try another canister with a tighter-fitting lid.


All you need is a zip-lock sandwich bag and a paper towel: (Do this experiment outdoors or in the kitchen sink and always wear safety glasses!)
- Place one and a half tablespoons of baking soda in the center of a paper towel and fold up the towel into a square package to hold the powder inside.
- Into the zip-lock sandwich bag add half a cup of vinegar and a quarter cup of warm water.
- Put the paper towel package into the mouth of the zip-lock bag and hold it out of the vinegar by pinching the sides of the plastic bag.
- Zip the bag closed then let the paper towel drop into the vinegar.
The bag will fill up with carbon dioxide gas and pop with a bang, so make sure it's in a sink or outside.
The foam and fizz that results from the baking soda and vinegar reaction can be used to demonstrate the eruption of a volcano.
You will need a model of a volcano made from plasticine or plaster of paris which has a well in the middle to hold a container in which the reagents can be placed.
- Place two tablespoons of baking soda in the container located in the volcano well.
- To a quarter cup of warm water add a few drops of a dark food coloring agent (to make the demo more realistic) and add that to the container holding the baking soda.
- Add half a cup of vinegar and watch the volcano erupt.
You will need lots of paper towels to clean up the mess once this demonstration is over.
It is a lot easier to start with a kit such as the 4M Volcano Making Kit

A common way to do this is to stretch a balloon, pre-filled with a certain amount of baking soda, over a clean, plastic water bottle in which some vinegar has been added. Tipping up the balloon causes the baking soda to mix with the vinegar and the resulting reaction gas pumps up the balloon.
Balloons expand to different extents depending on how much carbon dioxide gas is produced and this depends on the quantity of baking soda and vinegar used.
- Place 1 teaspoon baking soda (4g sodium bicarbonate) in the balloon.
- Add 4 tablespoons of vinegar ( 60 ml of 5% acetic acid) into a 1 liter water bottle.
- Stretch the mouth of the balloon over the mouth of the bottle then turn the balloon completely upright so that the baking soda inside the balloon pours into the vinegar.
Compare the size of this balloon to one expanded with:
- only half the amount of baking soda and vinegar or
- half the amount of baking soda added to the same amount of vinegar.


The reaction between baking soda and vinegar can be followed by analyzing the change in temperature, pH, mass and other parameters as the reaction progresses to completion.
To do this accurately requires some fairly expensive lab equipment connected to a computer running dedicated software, so it's not something you can do at home!
Here is an example of what you would find if you measured the reaction's temperature as time progressed:
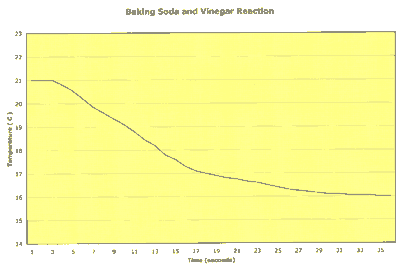
Notice that the reaction was finished in much less than a minute and that the reaction temperature decreased with time. This is an example of an endothermic reaction, one that needs heat to make it happen.