Vinegar Titration
The Best Way to Determine
Acetic Acid Content
"Step by step procedure for determing
the amount of acetic acid in vinegar."
Vinegar titration is a process which measures the volume of base (a standard solution of sodium hydroxide) required to completely neutralize the acetic acid in the vinegar sample.
Complete neutralization occurs when the color of the test solution changes from white to pink. From the amount of base used, the percent of acid in the vinegar can be accurately calculated.
Many local wine and beer making shops sell inexpensive titration test kits for determining the acidity in a wine, because the correct amount of acid improves the taste and inhibits harmful bacteria.
This acid titration test kit can be used for home vinegar making, but since the type of acid is different and the amount is typically ten times greater, the standard test procedure supplied with the wine test kit is not applicable for vinegar titration.
Instead use the step-by-step vinegar titration procedure outlined below, along with the equipment in the standard wine test kit to determine the amount of acetic acid in your homemade vinegar sample.
Equipment in the Acid Titration Kit for Wine
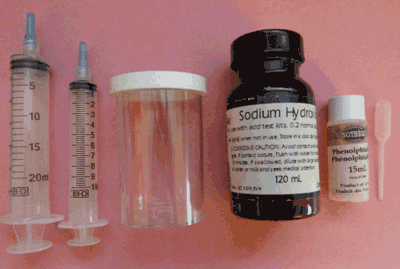
- - 20 ml syringe
- - 10 ml syringe
- - 150 ml plastic test container
- - 100 ml bottle of standard base (0.2 N sodium hydroxide)
- - 15 ml bottle of indicator solution with dropper (phenolphthalein)
Vinegar Titration Procedure
With the 10 ml syringe, measure out and add to the test container 2.0 ml of homemade vinegar. Then clean out the syringe.
With the 20 ml syringe, add 20 ml of water to the test container.
Add 3 drops of indicator solution to the test container.
Fill the cleaned 10 ml syringe to the 10 ml mark with the standard base.
Dispense the standard base solution slowly into the test container one drop at a time. As you add the base, gently swirl the test container to continuously mix its contents, and stop adding base when the vinegar solution turns pink.When the sample has changed color, note the amount of base solution used in the 10 ml syringe.
- (If you started at 10 ml in the syringe and finished at 1.6 ml then the amount of base used would be: 10.0 - 1.6 = 8.4 ml.)
Calculate the % acetic acid in the vinegar sample by multiplying the volume of base used by 0.6.
Example: If 8.4 ml of standard base was used to titrate the vinegar sample then the amount of acetic acid in the sample would be: 8.4 x 0.6 = 5.0%
You Tube video.